News
Blog & News
Blog & News
Sign-up for our newsletter
Thank you for contacting us.
We will get back to you as soon as possible.
We will get back to you as soon as possible.
Oops, there was an error sending your message.
Please try again later.
Please try again later.
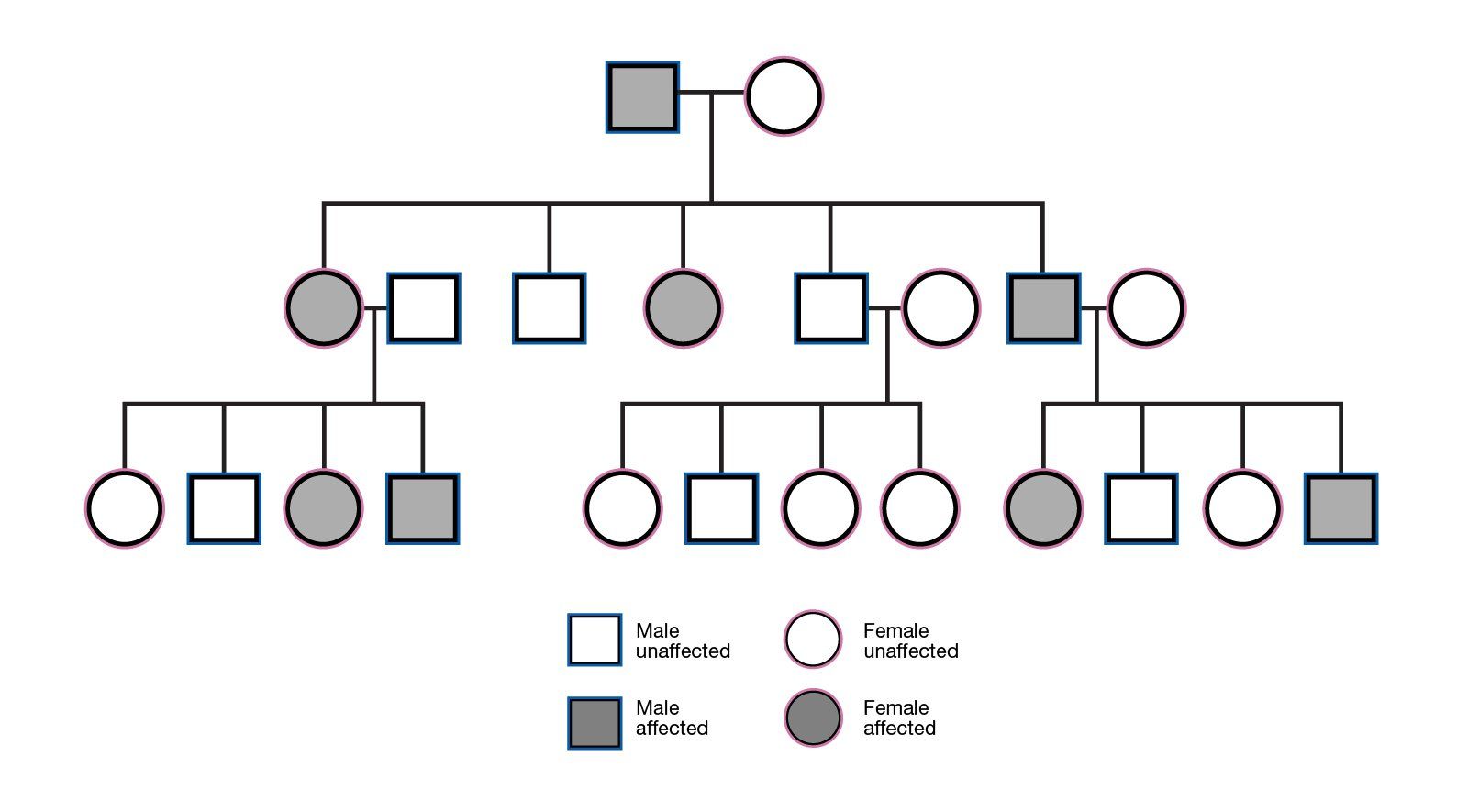
Informed consent failures: National Health Service Resolution data
📣Hot off the press - our analysis of NHS Resolution data regarding 'Fail to Warn' over the last decade, has been published in British Journal of Surgery.
We observe ‘Fail to warn - Informed consent’ litigation presents a common and increasing cost for NHS England. We also observe significant variation between Trusts and by specialty.
Further research is warranted to examine the reasons for changes in the frequency of litigation over time and whether variation in litigation is warranted between Trusts and by specialty. It would also be interesting to compare claims and costs from other jurisdictions worldwide. Deficiencies in the consent process may explain unwarranted variation, and processes to standardize and improve the quality of consent should be considered and evaluated.
To learn more, see: https://doi.org/10.1093/bjs/znad131
8th June 2023
What is 'health economics?!'
Being able to effectively communicate: why, what, and how we work in health economics is essential. Working in silos means that there are fewer opportunities for creative solutions and joined-up thinking.
At QC Medica we recognise the importance of breaking down silos to help people collaborate across boundaries. That's why over the past semester it has been exciting to host three teams of #ULMS #MBA students from the University of Liverpool tasked with creating new ideas for how we can better translate our purpose, research approach, and study findings.
We wish to congratulate all teams on their great work and share our best wishes in their future endeavours!
30th May 2023
How can we reduce health inequalities?
Health inequalities can be experienced by people grouped by different factors including:
- socioeconomic status and deprivation
- sharing certain protected characteristics
- belonging to vulnerable or excluded groups of society
- geography
The equitable implementation of NICE recommendations ensures that care provided is effective, makes efficient use of resources and reduces inequalities and unwarranted variation.
NICE has developed a series of resources to learn more about their activities and how NICE has been working to address health inequalities.
To learn more, see: https://www.nice.org.uk/about/what-we-do/nice-and-health-inequalities
7th January 2023
Happy thanksgiving 2022
Happy Thanksgiving to our friends and colleagues in the USA!
Wishing you a harvest of blessings, good health and good times!
24th November 2022
QC Medica at ISPOR Europe 2022
Our team are looking forward to attending ISPOR EU 2022
To meet our team, please contact: hello@qcmedica.com
25th October 2022
Today NICE launch their real-world evidence framework!
NICE, wish to make greater use of real-world data to resolve gaps in knowledge and drive forward access to innovations. The real-world evidence framework was developed to help resolve knowledge gaps and drive forward patient access to innovative treatments by:
- Identifying where real-world data can be used to reduce uncertainties and improve guidance
- Clearly describing best-practices for the planning, conduct, and reporting of real-world evidence studies to improve the quality and transparency of evidence
23rd June 2022
19th June 2022 is World Sickle Cell Day !
Sickle cell disease is the name for a group of inherited health conditions that affect the red blood cells. Sickle cell disease is a serious and lifelong health condition, although treatment can help manage many of the symptoms.
The main symptoms of sickle cell disease are:
- Painful episodes called sickle cell crises, which can be very severe and last up to a week
- Increased risk of serious infections
- Anaemia , which can cause tiredness and shortness of breath
Some people also experience other problems, such as delayed growth, strokes and lung problems.
For further information, see: https://www.sicklecellsociety.org/
19th June 2022
ISPOR 2022
Super few days at ISPOR—The Professional Society for Health Economics and Outcomes Research.
Our team participated in a workshop discussion on ‘challenges and opportunities in measuring and valuing health-related quality of life in rare genetic diseases affecting paediatric populations’
For further information about the even, see: https://www.ispor.org/conferences-education/conferences/past-conferences/ispor-2022
18th May 2022
Celebrating World Thalassaemia Day 2022!
Thalassaemia is an inherited and chronic condition that affects haemoglobin. People with thalassaemia produce either no or too little haemoglobin, which is used by red blood cells to carry oxygen around the body.
Most people born with thalassaemia experience health problems from a few months after birth. The main health problems associated with thalassaemia are:
- Anaemia: severe tiredness, weakness, shortness of breath, pounding, fluttering or palpitations
- Too much iron in the body: this is caused by the regular blood transfusions used to treat anaemia and can cause problems with the heart, liver and hormone levels if untreated
- Some people may also have delayed growth, weak and fragile bones (osteoporosis), and reduced fertility
For further information, see: https://ukts.org/
8th May 2022
Rare disesease day 2022
The future of living with a rare disease should not be left to luck!
This #RareDiseaseDay we come together to advocate for more research, better treatments and improved quality of life for all people with rare diseases.
To learn more, see: https://www.rarediseaseday.org/news/press-release-share-your-colours-for-rare-disease-day/
28th February 2022
Updated Consolidated Health Economic Evaluation Reporting Standard Checklist (CHEERS) 2022 checklist now available!
CHEERS 2022 has just been published in Value in Health! The new, expanded Consolidated Health Economic Evaluation Reporting Standards report provides guidance to improve the reporting of economic evaluations of health interventions.
For further information, see: https://www.ispor.org/heor-resources/good-practices/cheers
11th January 2022
Findings and recommendations into care for people with #sicklecell in the UK
Report published today by All Party Parliamentary Group on Sickle Cell and Thalassaemia (SCTAPPG) found:
- Sub-standard care on general wards and in A&E
- Failings in providing joined-up sickle cell care
- Low awareness of sickle cell among HCPs and inadequate training
- Negative attitudes towards sickle cell patients
- Inadequate investment in sickle cell care
For further information, see: https://www.sicklecellsociety.org/wp-content/uploads/2021/11/No-Ones-Listening-PDF-Final.pdf
15th November 2021
More research needed to understand the effectiveness of vaccines against emerging variants of concern
Early data from a single study has found the Pfizer vaccine found to be 88% effective in stopping symptomatic disease from the 'Indian' variant two weeks after the second dose, compared with 93% effectiveness against the Kent variant. The AstraZeneca vaccine was 60% effective against the 'Indian' variant, compared with 66% against the 'Kent' variant.
Public Health England (PHE) have stated the difference in effectiveness between the vaccines after two doses may be explained by the fact that rollout of second doses of AstraZeneca was later than for the Pfizer vaccine, which was approved first.
For more information, see: https://www.bbc.co.uk/news/uk-57214596
22nd May 2021
New clinical trial portal: ScanMedicine provides open access to global registrations of past, current and planned clinical research
ScanMedicine identifies information from 11 of the world’s leading clinical trial databases to provide open access to updated information on research conducted, ongoing, and what new medicines, devices and diagnostics are on the horizon.
This website research portal offers a great resources for investigators into the existing clinical research landscape
To explore further, see: https://scanmedicine.com/
18th May 2021
Today NICE launch their real-world evidence framework!
NICE, wish to make greater use of real-world data to resolve gaps in knowledge and drive forward access to innovations. The real-world evidence framework was developed to help resolve knowledge gaps and drive forward patient access to innovative treatments by:
- Identifying where real-world data can be used to reduce uncertainties and improve guidance
- Clearly describing best-practices for the planning, conduct, and reporting of real-world evidence studies to improve the quality and transparency of evidence
To learn more, see: :
https://www.nice.org.uk/corporate/ecd9/chapter/overview
23rd June 2022
Celebrating World Thalassaemia Day 2022!
Sickle cell disease is the name for a group of inherited health conditions that affect the red blood cells. Sickle cell disease is a serious and lifelong health condition, although treatment can help manage many of the symptoms.
The main symptoms of sickle cell disease are:
- Painful episodes called sickle cell crises, which can be very severe and last up to a week
- Increased risk of serious infections
- Anaemia , which can cause tiredness and shortness of breath
Some people also experience other problems, such as delayed growth, strokes and lung problems.
For further information, see: https://www.sicklecellsociety.org/
19th June 2022
ISPOR 2022
Super few days at ISPOR—The Professional Society for Health Economics and Outcomes Research.
Our team participated in a workshop discussion on ‘challenges and opportunities in measuring and valuing health-related quality of life in rare genetic diseases affecting paediatric populations’
For further information about the even, see: https://www.ispor.org/conferences-education/conferences/past-conferences/ispor-2022
18th May 2022
Celebrating World Thalassaemia Day 2022!
Thalassaemia is an inherited and chronic condition that affects haemoglobin. People with thalassaemia produce either no or too little haemoglobin, which is used by red blood cells to carry oxygen around the body.
Most people born with thalassaemia experience health problems from a few months after birth. The main health problems associated with thalassaemia are:
- Anaemia: severe tiredness, weakness, shortness of breath, pounding, fluttering or palpitations
- Too much iron in the body: this is caused by the regular blood transfusions used to treat anaemia and can cause problems with the heart, liver and hormone levels if untreated
- Some people may also have delayed growth, weak and fragile bones (osteoporosis), and reduced fertility
For further information, see: https://ukts.org/
8th May 2022
Rare disesease day 2022
The future of living with a rare disease should not be left to luck!
This #RareDiseaseDay we come together to advocate for more research, better treatments and improved quality of life for all people with rare diseases.
To learn more, see: https://www.rarediseaseday.org/news/press-release-share-your-colours-for-rare-disease-day/
28th February 2022
Updated Consolidated Health Economic Evaluation Reporting Standard Checklist (CHEERS) 2022 checklist now available!
CHEERS 2022 has just been published in Value in Health! The new, expanded Consolidated Health Economic Evaluation Reporting Standards report provides guidance to improve the reporting of economic evaluations of health interventions.
For further information, see: https://www.ispor.org/heor-resources/good-practices/cheers
11th January 2022
Findings and recommendations into care for people with #sicklecell in the UK
Report published today by All Party Parliamentary Group on Sickle Cell and Thalassaemia (SCTAPPG) found:
- Sub-standard care on general wards and in A&E
- Failings in providing joined-up sickle cell care
- Low awareness of sickle cell among HCPs and inadequate training
- Negative attitudes towards sickle cell patients
- Inadequate investment in sickle cell care
For further information, see: https://www.sicklecellsociety.org/wp-content/uploads/2021/11/No-Ones-Listening-PDF-Final.pdf
15th November 2021
More research needed to understand the effectiveness of vaccines against emerging variants of concern
Early data from a single study has found the Pfizer vaccine found to be 88% effective in stopping symptomatic disease from the 'Indian' variant two weeks after the second dose, compared with 93% effectiveness against the Kent variant. The AstraZeneca vaccine was 60% effective against the 'Indian' variant, compared with 66% against the 'Kent' variant.
Public Health England (PHE) have stated the difference in effectiveness between the vaccines after two doses may be explained by the fact that rollout of second doses of AstraZeneca was later than for the Pfizer vaccine, which was approved first.
For more information, see: https://www.bbc.co.uk/news/uk-57214596
22nd May 2021
New clinical trial portal: ScanMedicine provides open access to global registrations of past, current and planned clinical research
ScanMedicine identifies information from 11 of the world’s leading clinical trial databases to provide open access to updated information on research conducted, ongoing, and what new medicines, devices and diagnostics are on the horizon.
This website research portal offers a great resources for investigators into the existing clinical research landscape
To explore further, see: https://scanmedicine.com/
18th May 2021
May turns 'Purple for Stroke'
Make May Purple for Stroke is an annual awareness campaign by the Stroke Association.
The aims are to raise awareness about stroke and their impact on sufferers and their friends and families. Further, the campaign aims to raise awareness about the signs and symptoms of strokes, what causes strokes, what happens during a stroke and what to do in the event of someone suffering a stroke.
For more information or to get involved visit: https://www.stroke.org.uk/
10th May 2021
Join webinar for 'IMPACT HTA WP10 Appraisal Framework for Rare Diseases'
Join webinar on 12th May at 1pm for the launch of 'IMPACT HTA WP10 Appraisal Framework for Rare Diseases.' This webinar will be hosted by NICE and @iMPACT_HTA and include discussions with an international multi-stakeholder panel.
This framework aims to support robust, accountable decision-making across Europe on high-cost products for rare diseases that have a limited evidence base with higher uncertainty and intense stakeholder scrutiny.
Register here: https://bit.ly/2QGOwFU
4th May 2021
May turns 'Purple for Stroke'
Make May Purple for Stroke is an annual awareness campaign by the Stroke Association.
The aims are to raise awareness about stroke and their impact on sufferers and their friends and families. Further, the campaign aims to raise awareness about the signs and symptoms of strokes, what causes strokes, what happens during a stroke and what to do in the event of someone suffering a stroke.
For more information or to get involved visit: https://www.stroke.org.uk/
10th May 2021
Join webinar for 'IMPACT HTA WP10 Appraisal Framework for Rare Diseases'
Join webinar on 12th May at 1pm for the launch of 'IMPACT HTA WP10 Appraisal Framework for Rare Diseases.' This webinar will be hosted by NICE and @iMPACT_HTA and include discussions with an international multi-stakeholder panel.
This framework aims to support robust, accountable decision-making across Europe on high-cost products for rare diseases that have a limited evidence base with higher uncertainty and intense stakeholder scrutiny.
Register here: https://bit.ly/2QGOwFU
4th May 2021
Join PREFER webinar on the value of patient preferences to inform medical decision-making
What is a patient preference and what is the difference between a preference and patient-reported outcomes?
Presenters will set the scene for presentations on the value of patient preference studies for different stakeholders, explaining the role this input can have in regulatory decision making, and value for decision-making by health technology assessors and payers.
The webinar is due to take place on 26th April, to join or learn more, see: https://www.imi-prefer.eu/news/news-item/?tarContentId=940916
19th April 2021
Developing a health economics analysis plan (HEAP) to conduct a trial-based economic evaluation
Health economics analysis plans (HEAPs) support the robustness of Trial-Based Economic Evaluations.
Economic evaluations are frequently conducted alongside RCTs to inform funding allocation decisions. However, HEAPs are not universally implemented and as such, an Expert Delphi Consensus survey has been conducted to identify core items that were considered essential for inclusion within a HEAP.
To learn more about developing a HEAP and core items for consideration, see: https://www.valueinhealthjournal.com/action/showPdf?pii=S1098-3015%2820%2934444-2
13th April 2021
Save the date for 2021 EUnetHTA forum - 15th April 2021!
To participate go to the following link and use the password ‘eunethta21@online’. See: https://www.eventbrite.co.uk/e/2021-eunethta-forum-online-registration-132221775891
EUnetHTA supports collaboration between European HTA organisations that brings added value to healthcare systems at the European, national, and regional level. Through its activities, EUnetHTA:
- Supports efficient production and use of HTA in countries across Europe.
- Provides an independent and science-based platform for HTA agencies in countries across Europe to exchange and develop HTA information and methodology.
- Provides an access point for communication with stakeholders to promote transparency, objectivity, independence of expertise, fairness of procedure and appropriate stakeholder consultations.
- Develops alliances with contributing fields of research to support a stronger and broader evidence base for HTA while using the best available scientific competence.
28th March 2021
World Tuberculosis (TB) Day - 24th March 2021
Each year we commemorate World Tuberculosis (TB) Day on the 24th of March to raise awareness about the devastating health, social and economic consequences of TB.
The date marks the day in 1882 when Dr Robert Koch announced that he had discovered the bacterium that causes TB, which opened the way towards diagnosing and curing this disease. TB remains one of the world’s deadliest infectious killers. Each day, nearly 4000 lose their lives to TB and close to 28,000 people become ill with this preventable and curable disease. Global efforts to combat TB have saved an estimated 63 million lives since the year 2000.
The theme of World TB Day 2021 - ‘The Clock is Ticking’ which conveys the sense that the world is running out of time to act on the commitments to end TB made by global leaders.
To learn more, see: https://www.who.int/campaigns/world-tb-day
24th March 2021
Save the date for 2021 EUnetHTA forum - 15th April 2021!
To participate go to the following link and use the password ‘eunethta21@online’. See: https://www.eventbrite.co.uk/e/2021-eunethta-forum-online-registration-132221775891
EUnetHTA supports collaboration between European HTA organisations that brings added value to healthcare systems at the European, national, and regional level. Through its activities, EUnetHTA:
- Supports efficient production and use of HTA in countries across Europe.
- Provides an independent and science-based platform for HTA agencies in countries across Europe to exchange and develop HTA information and methodology.
- Provides an access point for communication with stakeholders to promote transparency, objectivity, independence of expertise, fairness of procedure and appropriate stakeholder consultations.
- Develops alliances with contributing fields of research to support a stronger and broader evidence base for HTA while using the best available scientific competence.
28th March 2021
World Tuberculosis (TB) Day - 24th March 2021
Each year we commemorate World Tuberculosis (TB) Day on the 24th of March to raise awareness about the devastating health, social and economic consequences of TB.
The date marks the day in 1882 when Dr Robert Koch announced that he had discovered the bacterium that causes TB, which opened the way towards diagnosing and curing this disease. TB remains one of the world’s deadliest infectious killers. Each day, nearly 4000 lose their lives to TB and close to 28,000 people become ill with this preventable and curable disease. Global efforts to combat TB have saved an estimated 63 million lives since the year 2000.
The theme of World TB Day 2021 - ‘The Clock is Ticking’ which conveys the sense that the world is running out of time to act on the commitments to end TB made by global leaders.
To learn more, see: https://www.who.int/campaigns/world-tb-day
24th March 2021
Join ISPOR's Science Strategy webinar tomorrow!
Join ISPOR's Science Strategy webinar tomorrow!
ISPOR's Science Strategy is intended to guide conference themes, task forces, initiatives, and other scientific activitiesto help meet the evolving needs of healthcare decision making.
The webinar will review theme areas and focal points, how the themes were generated, and potential implications for future ISPOR activities.
To register for the ISPOR's Science Strategy webinar, see: https://www.ispor.org/conferences-education/calendar/event/2021/03/23/default-calendar/ispors-new-science-strategy---a-member-generated-roadmap-of-heor-priorities-and-frontiers?utm_medium=social_media&utm_source=linkedin&utm_campaign=educational_webinar&utm_content=webinar_science_strategoes_mar22&utm_term=heor+healthcare
23rd March 2021
Today is Young Carers Action Day
Today is Young Carers Action Day, which is a nationwide event organised by the Carer’s Trust aimed at raising awareness of the struggles and importance of young carers across the country.
As many as 1 in 5 secondary school-aged children have been or will be put in the position of a young carer, often being responsible for a family member who is ill, disabled or incapacitated. As a consequence, many suffer from issues relating to caring at such a young age, including a damaged social life, isolation and reduced educational progression. As such, we advocate for consideration of wider societal impacts associated with disease burden within health technology assessments (HTA).
To learn more about Young Carers Action Day, see: https://carers.org/young-carers-action-day-2021/young-carers-action-day-2021-resources
16th March 2021
Join ISPOR's Science Strategy webinar tomorrow!
Join ISPOR's Science Strategy webinar tomorrow!
ISPOR's Science Strategy is intended to guide conference themes, task forces, initiatives, and other scientific activitiesto help meet the evolving needs of healthcare decision making.
The webinar will review theme areas and focal points, how the themes were generated, and potential implications for future ISPOR activities.
To register for the ISPOR's Science Strategy webinar, see: https://www.ispor.org/conferences-education/calendar/event/2021/03/23/default-calendar/ispors-new-science-strategy---a-member-generated-roadmap-of-heor-priorities-and-frontiers?utm_medium=social_media&utm_source=linkedin&utm_campaign=educational_webinar&utm_content=webinar_science_strategoes_mar22&utm_term=heor+healthcare
23rd March 2021
Today is Young Carers Action Day
Today is Young Carers Action Day, which is a nationwide event organised by the Carer’s Trust aimed at raising awareness of the struggles and importance of young carers across the country.
As many as 1 in 5 secondary school-aged children have been or will be put in the position of a young carer, often being responsible for a family member who is ill, disabled or incapacitated. As a consequence, many suffer from issues relating to caring at such a young age, including a damaged social life, isolation and reduced educational progression. As such, we advocate for consideration of wider societal impacts associated with disease burden within health technology assessments (HTA).
To learn more about Young Carers Action Day, see: https://carers.org/young-carers-action-day-2021/young-carers-action-day-2021-resources
16th March 2021
March is Ovarian Cancer Awareness Month
March is Ovarian Cancer Awareness Month.
Ovarian cancer is the biggest gynaecological cause of death in the in the UK. Early diagnoses is associated with improved health outcomes and survival.
You can find out more about how to get involved in Ovarian Cancer Awareness Month, as well as read the personal stories of women living with and beyond an ovarian cancer diagnosis, at: https://ovarian.org.uk/march-ovarian-cancer-awareness-month/
9th March 2021
Join the NICE 5-year strategy virtual event launch
Join the 5-year strategy virtual event launch of the National Institute for Health and Care Excellence (NICE) to find out more about how NICE plan to develop their products, processes, partnerships and people.
The launch event aims to review how NICE plan to:
- Provide faster, flexible evaluation of new medicines and innovative technologies. This includes digital health tech.
- Produce living guidelines. This is so clinicians and practitioners can be confident that our advice is up to date and incorporates new technologies.
- Improve access to our guidance. Making it easier to find our recommendations and the evidence behind them.
- Help remove barriers to adoption of new technologies, including funding streams so benefits are seen more quickly.
- Use real world data routinely in all our work.
- Foster innovative ways of listening to patient and public opinion.
- Drive the future research agenda to make sure it addresses priority questions for NICE and patients.
The virtual launch event is due to take place: Monday, 19th April 2021, 11-12:15 GMT. Follow this link to register for free: https://www.nice.org.uk/news/events/5-year-strategy-virtual-event-launch
10th March 2021
March is Ovarian Cancer Awareness Month
March is Ovarian Cancer Awareness Month.
Ovarian cancer is the biggest gynaecological cause of death in the in the UK. Early diagnoses is associated with improved health outcomes and survival.
You can find out more about how to get involved in Ovarian Cancer Awareness Month, as well as read the personal stories of women living with and beyond an ovarian cancer diagnosis, at: https://ovarian.org.uk/march-ovarian-cancer-awareness-month/
9th March 2021
Join the NICE 5-year strategy virtual event launch
Join the 5-year strategy virtual event launch of the National Institute for Health and Care Excellence (NICE) to find out more about how NICE plan to develop their products, processes, partnerships and people.
The launch event aims to review how NICE plan to:
- Provide faster, flexible evaluation of new medicines and innovative technologies. This includes digital health tech.
- Produce living guidelines. This is so clinicians and practitioners can be confident that our advice is up to date and incorporates new technologies.
- Improve access to our guidance. Making it easier to find our recommendations and the evidence behind them.
- Help remove barriers to adoption of new technologies, including funding streams so benefits are seen more quickly.
- Use real world data routinely in all our work.
- Foster innovative ways of listening to patient and public opinion.
- Drive the future research agenda to make sure it addresses priority questions for NICE and patients.
The virtual launch event is due to take place: Monday, 19th April 2021, 11-12:15 GMT. Follow this link to register for free: https://www.nice.org.uk/news/events/5-year-strategy-virtual-event-launch
10th March 2021
Complex Needs: Mental Health Research Collaboration
Great collaboration with colleagues from Liverpool John Moores University and Cheshire & Wirral Partnership NHS Foundation Trust to examine outcomes associated with alternative care pathways for people with complex mental health needs.
To learn more about this ongoing research, see:
9th March 2021
International Women's Day: 8th March 2021
At QC Medica we support International Women’s Day. In line with this year’s #choosetochallenge theme, we are committed to taking action for equality. We will continue to #choosetochallenge the roots of inequality.
To learn more about IWD, see:
#choosetochallange #IWD2021 #diversityintheworkplace #genderequality
8th March 2021
Complex Needs: Mental Health Research Collaboration
Great collaboration with colleagues from Liverpool John Moores University and Cheshire & Wirral Partnership NHS Foundation Trust to examine outcomes associated with alternative care pathways for people with complex mental health needs.
To learn more about this ongoing research, see:
9th March 2021
International Women's Day: 8th March 2021
At QC Medica we support International Women’s Day. In line with this year’s #choosetochallenge theme, we are committed to taking action for equality. We will continue to #choosetochallenge the roots of inequality.
To learn more about IWD, see:
#choosetochallange #IWD2021 #diversityintheworkplace #genderequality
8th March 2021
Real World Evidence: Bridging the Gap in HTA
There is a growing interest in real world evidence (RWE) to assess the value of health interventions:
1) to examine real-world outcomes, and
2) to support evidence generation in the context of limited trial data.
A recent study examining the 'Use of real-world evidence in economic assessments of pharmaceuticals in the United States' found that RWE data were included in approximately 1/3 of economic model inputs from Institute for Clinical and Economic Review, and RWE usage is increasing.
To learn more about RWE, please see:
2nd March 2021
Eating Disorders Awareness Week: 1st-7th March 2021
Eating Disorders Awareness Week starting today was established to raise awareness and understanding of eating disorders. Approximately 1.25 million people in the UK are living with an eating disorder right now.
Eating disorders are serious mental illnesses affecting people of all ages, genders, ethnicities and backgrounds. People with eating disorders use disordered eating behaviour as a way to cope with difficult situations or feelings. This behaviour can include limiting the amount of food eaten, eating very large quantities of food at once, getting rid of food eaten through unhealthy means, or a combination of these behaviours.
It’s important to remember that eating disorders are not all about food itself, but about feelings. An eating disorder is never the fault of the person experiencing it, and anyone who has an eating disorder deserves fast support to help them get better. Types of eating disorders include: anorexia , ARFID, binge eating disorder, bulimia, OSFED (other specified feeding or eating disorder).
To learn more, see:
1st March 2021
Real World Evidence: Bridging the Gap in HTA
There is a growing interest in real world evidence (RWE) to assess the value of health interventions:
1) to examine real-world outcomes, and
2) to support evidence generation in the context of limited trial data.
A recent study examining the 'Use of real-world evidence in economic assessments of pharmaceuticals in the United States' found that RWE data were included in approximately 1/3 of economic model inputs from Institute for Clinical and Economic Review, and RWE usage is increasing.
To learn more about RWE, please see:
2nd March 2021
Eating Disorders Awareness Week: 1st-7th March 2021
Eating Disorders Awareness Week starting today was established to raise awareness and understanding of eating disorders. Approximately 1.25 million people in the UK are living with an eating disorder right now.
Eating disorders are serious mental illnesses affecting people of all ages, genders, ethnicities and backgrounds. People with eating disorders use disordered eating behaviour as a way to cope with difficult situations or feelings. This behaviour can include limiting the amount of food eaten, eating very large quantities of food at once, getting rid of food eaten through unhealthy means, or a combination of these behaviours.
It’s important to remember that eating disorders are not all about food itself, but about feelings. An eating disorder is never the fault of the person experiencing it, and anyone who has an eating disorder deserves fast support to help them get better. Types of eating disorders include: anorexia , ARFID, binge eating disorder, bulimia, OSFED (other specified feeding or eating disorder).
To learn more, see:
1st March 2021
World Encephalitis Day: 22nd February 2021
Encephalitis is an inflammation of the brain. It is caused either by an infection invading the brain (infectious encephalitis) or through the immune system attacking the brain in error (post-infectious or autoimmune encephalitis). Encephalitis can be caused by viral infections (including the herpes simplex virus and the chickenpox virus), a problem with the immune system, bacterial or fungal infections, and some types of encephalitis are spread by mosquitoes, ticks and mammals .
World Encephalitis Day is the global awareness day for people who have been directly or indirectly affected by encephalitis. Encephalitis requires urgent treatment and it is important to increase global awareness of symptoms to save lives, including: flu-like symptoms, disorientation, seizures or fits, changes in personality and behaviour
difficulty speaking, body movement weakness, and loss of consciousness.
To learn more about Encephalitis, please see:
and:
22nd February 2021
We support LGBT+ History Month: February 2021
At QC Medica we support LGBT+ History Month.
This initiative was created in the UK by Schools Out UK and first took place in February 2005. The project aims to make February an informative and celebratory month, to educate out prejudice and make LGBT+ people, in all their rich diversity, visible.
To find out more, please visit:
17th February 2021
World Encephalitis Day: 22nd February 2021
Encephalitis is an inflammation of the brain. It is caused either by an infection invading the brain (infectious encephalitis) or through the immune system attacking the brain in error (post-infectious or autoimmune encephalitis). Encephalitis can be caused by viral infections (including the herpes simplex virus and the chickenpox virus), a problem with the immune system, bacterial or fungal infections, and some types of encephalitis are spread by mosquitoes, ticks and mammals .
World Encephalitis Day is the global awareness day for people who have been directly or indirectly affected by encephalitis. Encephalitis requires urgent treatment and it is important to increase global awareness of symptoms to save lives, including: flu-like symptoms, disorientation, seizures or fits, changes in personality and behaviour
difficulty speaking, body movement weakness, and loss of consciousness.
To learn more about Encephalitis, please see:
and:
22nd February 2021
We support LGBT+ History Month: February 2021
At QC Medica we support LGBT+ History Month.
This initiative was created in the UK by Schools Out UK and first took place in February 2005. The project aims to make February an informative and celebratory month, to educate out prejudice and make LGBT+ people, in all their rich diversity, visible.
To find out more, please visit:
17th February 2021
Upcoming ISPOR 2021 conference
We are looking forward to attending #ISPOR2021 and learning about how we can address challenges and opportunities in HEOR, including:
- How can we further strengthen HEOR’s relevance, particularly moving beyond product assessment, to address systemic healthcare challenges?
- How should HEOR use its chance for renewal?
- What innovations in collaboration approaches are advancing HEOR beyond its traditional sphere of influence?
- Which upgrades in HEOR methodology, application, and practice are needed to future-proof HEOR impact on healthcare decision-making?
Virtual ISPOR 2021 will take place between 17-20th May. Follow the link to learn more and register:
15th February 2021
Upcoming ISPOR 2021 conference
We are looking forward to attending #ISPOR2021 and learning about how we can address challenges and opportunities in HEOR, including:
- How can we further strengthen HEOR’s relevance, particularly moving beyond product assessment, to address systemic healthcare challenges?
- How should HEOR use its chance for renewal?
- What innovations in collaboration approaches are advancing HEOR beyond its traditional sphere of influence?
- Which upgrades in HEOR methodology, application, and practice are needed to future-proof HEOR impact on healthcare decision-making?
Virtual ISPOR 2021 will take place between 17-20th May. Follow the link to learn more and register:
15th February 2021
International Day of Women and Girls in Science
Today is International Day of Women and Girls in Science and we're celebrating all the fantastic women working in health and social care research. Today we want to say thank you.
Thank you to all #WomenInScience, from the medical field, health sector, and chemistry, to biology.
To learn more, see:
11th February 2021
Join HTAi Webinar: HTA and Value Based Healthcare Methods
Join HTAi Webinar: HTA and Value Based Healthcare Methods
Thu, Mar 4, 2021 4:00 PM - 5:00 PM GMT
This webinar will be made up of 2 sessions. The first session, will discuss the methodological differences between HTA and Value-Based Healthcare and how they can be applied to Medical Devices. This session aims to provide participants with a comprehensive understanding of how HTA and VBHC methods can be used in practice. The webinar aims to further the goals and mission of the Medical Devices Interest Group, including advancing the dialogue on Medical Device HTA methods and evidence based policy-making.
Follow the link to sign-up and learn more:
10th February 2021
International Day of Women and Girls in Science
Today is International Day of Women and Girls in Science and we're celebrating all the fantastic women working in health and social care research. Today we want to say thank you.
Thank you to all #WomenInScience, from the medical field, health sector, and chemistry, to biology.
To learn more, see:
11th February 2021
Join HTAi Webinar: HTA and Value Based Healthcare Methods
Join HTAi Webinar: HTA and Value Based Healthcare Methods
Thu, Mar 4, 2021 4:00 PM - 5:00 PM GMT
This webinar will be made up of 2 sessions. The first session, will discuss the methodological differences between HTA and Value-Based Healthcare and how they can be applied to Medical Devices. This session aims to provide participants with a comprehensive understanding of how HTA and VBHC methods can be used in practice. The webinar aims to further the goals and mission of the Medical Devices Interest Group, including advancing the dialogue on Medical Device HTA methods and evidence based policy-making.
Follow the link to sign-up and learn more:
10th February 2021
NICE consultation for proposals for change to the processes used for health technology evaluation
NICE's consultation is open on proposals for change to the processes used for health technology evaluation at NICE.
The new proposals aim to:
-speed up patient access to new and promising health technologies
-support better market access
-simplify the health technology evaluation process.
The consultation process is open for feedback and comments until 11:59pm 15 April 2021.
To learn more and to provide feedback, see:
8th February 2021
New research publication on 'Potential approaches for the pricing of cancer medicines across Europe'
See our latest research collaboration on 'Potential approaches for the pricing of cancer medicines across Europe to enhance the sustainability of healthcare systems’ which has been published in Expert Review of Pharmacoeconomics & Outcomes Research.
A number of potential pricing approaches were discussed including minimum effectiveness levels for new cancer medicines, managed entry agreements, multicriteria decision analyses (MCDAs), differential/tiered pricing, fair pricing models, amortization models as well as de-linkage models. We anticipate that we are likely to see a growth in alternative pricing deliberations in view of ongoing challenges including the considerable number of new cancer medicines in development including gene therapies.
To read more, please see:
5th February 2021
NICE consultation for proposals for change to the processes used for health technology evaluation
NICE's consultation is open on proposals for change to the processes used for health technology evaluation at NICE.
The new proposals aim to:
-speed up patient access to new and promising health technologies
-support better market access
-simplify the health technology evaluation process.
The consultation process is open for feedback and comments until 11:59pm 15 April 2021.
To learn more and to provide feedback, see:
8th February 2021
New research publication on 'Potential approaches for the pricing of cancer medicines across Europe'
See our latest research collaboration on 'Potential approaches for the pricing of cancer medicines across Europe to enhance the sustainability of healthcare systems’ which has been published in Expert Review of Pharmacoeconomics & Outcomes Research.
A number of potential pricing approaches were discussed including minimum effectiveness levels for new cancer medicines, managed entry agreements, multicriteria decision analyses (MCDAs), differential/tiered pricing, fair pricing models, amortization models as well as de-linkage models. We anticipate that we are likely to see a growth in alternative pricing deliberations in view of ongoing challenges including the considerable number of new cancer medicines in development including gene therapies.
To read more, please see:
5th February 2021
World Cancer Day - 4th February 2021
Today is World Cancer Day - 4th February 2021 - Many cancers can be cured if diagnosed early and treated. This day aims to inform and encourage people on prevention, early diagnosis, and to increase access treatment.
The 2021 theme is "I AM and I Will. Together, all our actions matter." We encourage you to get involved and share the message.
To read more, please see
4th February 2021
Join us at HTAi 2021 where we will be discussing our research 'examining NICE patient access schemes and commercial arrangements'
Our team are delighted to announce that we will be presenting our research "Examining the implementation of NICE patient access schemes and commercial arrangements" at HTAi 2021.
Please follow the link to learn more about HTAi 2021 and to register:
4th February 2021
World Cancer Day - 4th February 2021
Today is World Cancer Day - 4th February 2021 - Many cancers can be cured if diagnosed early and treated. This day aims to inform and encourage people on prevention, early diagnosis, and to increase access treatment.
The 2021 theme is "I AM and I Will. Together, all our actions matter." We encourage you to get involved and share the message.
To read more, please see
4th February 2021
Join us at HTAi 2021 where we will be discussing our research 'examining NICE patient access schemes and commercial arrangements'
Our team are delighted to announce that we will be presenting our research "Examining the implementation of NICE patient access schemes and commercial arrangements" at HTAi 2021.
Please follow the link to learn more about HTAi 2021 and to register:
4th February 2021
New research published to examine 'The Budget Impact of Monoclonal Antibodies to Treat Metastatic Colorectal Cancer in Brazil
See our latest research collaboration on 'The Budget Impact of Monoclonal Antibodies Used to Treat Metastatic Colorectal Cancer in Minas Gerais, Brazil' which has recently been published.
A budget impact analysis was conducted to explore the impact of incorporating mAbs as first-line treatment for mCRC in Minas Gerais considering a 5 year time horizon.
To read more, please see
30th January 2021
Rare disease day to take place 28th February 2021!
Rare Disease Day takes place on 28th of February 2021 to raise awareness about rare diseases and their impact on patients' lives.
1 in 20 people will live with a rare disease at some point in their life. Many patients go undiagnosed and treatments need to be developed.
Please follow the link to learn more and share the message:
24th January 2021
New research published to examine 'The Budget Impact of Monoclonal Antibodies to Treat Metastatic Colorectal Cancer in Brazil
See our latest research collaboration on 'The Budget Impact of Monoclonal Antibodies Used to Treat Metastatic Colorectal Cancer in Minas Gerais, Brazil' which has recently been published.
A budget impact analysis was conducted to explore the impact of incorporating mAbs as first-line treatment for mCRC in Minas Gerais considering a 5 year time horizon.
To read more, please see
30th January 2021
Rare disease day to take place 28th February 2021!
Rare Disease Day takes place on 28th of February 2021 to raise awareness about rare diseases and their impact on patients' lives.
1 in 20 people will live with a rare disease at some point in their life. Many patients go undiagnosed and treatments need to be developed.
Please follow the link to learn more and share the message:
24th January 2021
Free Online Workshop to discuss 'Economic Evaluation in Mental Health'
The Evidence Synthesis Network have organised an online workshop to discuss 'Economic Evaluation in Mental Health' for the 24th Feb 2021 at 14:00 GMT.
The Evidence Synthesis Network brings together more than 190 health researchers, guideline developers and policy makers from across Greater Manchester and the North West with an interest in evidence synthesis in association with the National Institute for Health and Care Excellence (NICE).
The workshop is free to attend with registration required below:
20th January 2021
#QCMedica wishes you all the best for 2021 !
We would like to take this opportunity to thank our Clients, Colleagues, Partners, Friends and importantly, our Families who supported us in achieving so much in the past year. Our network is truly global and we look forward to exciting new collaborations in the new year.
Together we look forward to continuing to improve health care.
With best wishes,
Team – QC Medica
1st January 2021
Free Online Workshop to discuss 'Economic Evaluation in Mental Health'
The Evidence Synthesis Network have organised an online workshop to discuss 'Economic Evaluation in Mental Health' for the 24th Feb 2021 at 14:00 GMT.
The Evidence Synthesis Network brings together more than 190 health researchers, guideline developers and policy makers from across Greater Manchester and the North West with an interest in evidence synthesis in association with the National Institute for Health and Care Excellence (NICE).
The workshop is free to attend with registration required below:
20th January 2021
#QCMedica wishes you all the best for 2021 !
We would like to take this opportunity to thank our Clients, Colleagues, Partners, Friends and importantly, our Families who supported us in achieving so much in the past year. Our network is truly global and we look forward to exciting new collaborations in the new year.
Together we look forward to continuing to improve health care.
With best wishes,
Team – QC Medica
1st January 2021
New publication: Examining the uptake of predictive
BRCA
testing in the UK
The World Health Organisation (WHO) define 'health equity' as the absence of unfair and avoidable or remediable differences in health among population groups defined socially, economically, demographically or geographically.
Our research found that at least one relative of 83.4% of probands were BRCA
tested. Females and relatives of probands affected by cancer were more likely to undergo testing. Time-to-testing from proband to relative testing varied widely.
Differing rates of predictive BRCA
testing require additional research to examine whether these findings reflect differing health needs for predictive BRCA
testing or whether this reflects an inequality in access.
To read more, please follow the link below.
20th December 2020
NICE Methods Consultation
NICE have proposed changes to address a number of current challenges with treatment evaluation.
The proposals for change include the removal of the End of Life (EoL) modifier for QALY value, and the addition of a modifier for disease severity.
Another proposal for change includes changing the discount rate for both costs and health effects, going from 3.5%, as stated in the current methods guide, to 1.5%. The use of lower discount rates implies an increase in the value that NICE places on future costs and health benefits. A lower discount rate, as proposed in the consultation, would be of relevance for technologies that have high upfront costs and benefits that occur in the future and/or may last a lifetime e.g. curative therapies.
There are several important decisions yet to be made to ensure that these changes can be implemented. We look forward to the next round of consultation.
To read more, please follow the link below.
11th December 2020
New publication: Examining the uptake of predictive
BRCA
testing in the UK
The World Health Organisation (WHO) define 'health equity' as the absence of unfair and avoidable or remediable differences in health among population groups defined socially, economically, demographically or geographically.
Our research found that at least one relative of 83.4% of probands were BRCA
tested. Females and relatives of probands affected by cancer were more likely to undergo testing. Time-to-testing from proband to relative testing varied widely.
Differing rates of predictive BRCA
testing require additional research to examine whether these findings reflect differing health needs for predictive BRCA
testing or whether this reflects an inequality in access.
To read more, please follow the link below.
20th December 2020
NICE Methods Consultation
NICE have proposed changes to address a number of current challenges with treatment evaluation.
The proposals for change include the removal of the End of Life (EoL) modifier for QALY value, and the addition of a modifier for disease severity.
Another proposal for change includes changing the discount rate for both costs and health effects, going from 3.5%, as stated in the current methods guide, to 1.5%. The use of lower discount rates implies an increase in the value that NICE places on future costs and health benefits. A lower discount rate, as proposed in the consultation, would be of relevance for technologies that have high upfront costs and benefits that occur in the future and/or may last a lifetime e.g. curative therapies.
There are several important decisions yet to be made to ensure that these changes can be implemented. We look forward to the next round of consultation.
To read more, please follow the link below.
11th December 2020
COVID-19: Landscape overview of COVID-19 candidate vaccines
Last week, BioNTech and co-developers Pfizer said preliminary analysis showed their vaccine could prevent more than 90% of people from getting COVID-19.
The UK is expected to get 10 million doses of the BioNTech/Pfizer vaccine by the end of the year, with a further 30 million doses already ordered. With many vaccines in development and with early positive results from the BioNTech/Pfizer vaccine, we look forward to hearing more.
To read more about candidate COVID-19 vaccines in development, please follow the link below.
15th November 2020
Published research collaboration: Examining resource constraints and price increases for medicines and protection equipment for COVID-19 in Africa
Recent months have seen increases in utilisation of vitamins/ immune boosters and PPE. However, concerns with increased utilisation of antimicrobials needs addressing alongside misinformation, unintended consequences from the pandemic and any appreciable price rise. Community pharmacists and patient organisations can play key role in providing evidence-based advice, helping moderate prices through improved stock management, and helping address unintended consequences of the pandemic.
To read more, please follow the link below.
Sefah IA, Ogunleye OO, Essah DO et al. Rapid assessment of the potential paucity and price increases for suggested medicines and protection equipment for COVID-19 across developing countries with a particular focus on Africa and the implications. Front. Pharmacol. 2020. https://www.frontiersin.org/articles/10.3389/fphar.2020.588106/abstract
11th November 2020
COVID-19: Landscape overview of COVID-19 candidate vaccines
Last week, BioNTech and co-developers Pfizer said preliminary analysis showed their vaccine could prevent more than 90% of people from getting COVID-19.
The UK is expected to get 10 million doses of the BioNTech/Pfizer vaccine by the end of the year, with a further 30 million doses already ordered. With many vaccines in development and with early positive results from the BioNTech/Pfizer vaccine, we look forward to hearing more.
To read more about candidate COVID-19 vaccines in development, please follow the link below.
15th November 2020
Published research collaboration: Examining resource constraints and price increases for medicines and protection equipment for COVID-19 in Africa
Recent months have seen increases in utilisation of vitamins/ immune boosters and PPE. However, concerns with increased utilisation of antimicrobials needs addressing alongside misinformation, unintended consequences from the pandemic and any appreciable price rise. Community pharmacists and patient organisations can play key role in providing evidence-based advice, helping moderate prices through improved stock management, and helping address unintended consequences of the pandemic.
To read more, please follow the link below.
Sefah IA, Ogunleye OO, Essah DO et al. Rapid assessment of the potential paucity and price increases for suggested medicines and protection equipment for COVID-19 across developing countries with a particular focus on Africa and the implications. Front. Pharmacol. 2020. https://www.frontiersin.org/articles/10.3389/fphar.2020.588106/abstract
11th November 2020
ISPOR EU 2020 Virtual Conference
ISPOR Europe 2020 on 16-19 November 2020 is a virtual conference this year due to the COVID-19 pandemic and associated government regulations, restrictions and advisories.
We will keep you updated on any further announcements and schedule of the virtual programme.
2nd September 2020
Published research collaboration: Examining the response to COVID-19 pandemic across Africa
There are multiple activities across Africa to reduce the spread of COVID-19 and address misinformation, which can be catastrophic, assisted by the WHO and others, which appear to be working in a number of countries. Research is ongoing to clarify the unintended consequences given ongoing concerns to guide future activities. Countries are learning from each other.
To read more, please follow the link below.
OO, Basu D, Mueller D et al. Response to the novel corona virus (COVID-19) pandemic across Africa: successes, challenges and implications for the future. Front. Pharmacol. 2020. https://www.frontiersin.org/articles/10.3389/fphar.2020.01205/abstract
26th July 2020
ISPOR EU 2020 Virtual Conference
ISPOR Europe 2020 on 16-19 November 2020 is a virtual conference this year due to the COVID-19 pandemic and associated government regulations, restrictions and advisories.
We will keep you updated on any further announcements and schedule of the virtual programme.
2nd September 2020
Published research collaboration: Examining the response to COVID-19 pandemic across Africa
There are multiple activities across Africa to reduce the spread of COVID-19 and address misinformation, which can be catastrophic, assisted by the WHO and others, which appear to be working in a number of countries. Research is ongoing to clarify the unintended consequences given ongoing concerns to guide future activities. Countries are learning from each other.
To read more, please follow the link below.
OO, Basu D, Mueller D et al. Response to the novel corona virus (COVID-19) pandemic across Africa: successes, challenges and implications for the future. Front. Pharmacol. 2020. https://www.frontiersin.org/articles/10.3389/fphar.2020.01205/abstract
26th July 2020
ISPOR 2020 Virtual Conference
ISPOR have redesigned ISPOR 2020 the Society’s annual conference into Virtual ISPOR 2020 HEOR: Advancing Evidence to Action to take place over the original conference dates of May 18-20. All sessions are scheduled in Eastern Daylight Time (EDT).
The 3-day virtual conference offers an engaging 60 hours of content delivered by global HEOR experts and stakeholders. The scientific program for this virtual conference remains as dynamic and robust as was planned for the onsite version and will include 3 plenary sessions, issue panels, workshops, podium presentations, and sponsored symposia. Sessions will be presented in a variety of formats including live and recorded presentations with real-time interaction with presenters via Q and A.
18th May 2020
EURORDIS Rare Disease Day
Rare Disease Day is an observance held on the last day of February to raise awareness for rare diseases and improve access to treatment and medical representation for individuals with rare diseases and their families. The European Organisation for Rare Diseases (EURORDIS) established the day in 2008 to raise awareness for unknown or overlooked illnesses.
Treatment for many rare diseases is insufficient, as are the social networks to support individuals with rare diseases and their families.
Please follow the link to learn more about EURORDIS:
https://www.eurordis.org/
28th February 2020
ISPOR 2020 Virtual Conference
ISPOR have redesigned ISPOR 2020 the Society’s annual conference into Virtual ISPOR 2020 HEOR: Advancing Evidence to Action to take place over the original conference dates of May 18-20. All sessions are scheduled in Eastern Daylight Time (EDT).
The 3-day virtual conference offers an engaging 60 hours of content delivered by global HEOR experts and stakeholders. The scientific program for this virtual conference remains as dynamic and robust as was planned for the onsite version and will include 3 plenary sessions, issue panels, workshops, podium presentations, and sponsored symposia. Sessions will be presented in a variety of formats including live and recorded presentations with real-time interaction with presenters via Q and A.
18th May 2020
EURORDIS Rare Disease Day
Rare Disease Day is an observance held on the last day of February to raise awareness for rare diseases and improve access to treatment and medical representation for individuals with rare diseases and their families. The European Organisation for Rare Diseases (EURORDIS) established the day in 2008 to raise awareness for unknown or overlooked illnesses.
Treatment for many rare diseases is insufficient, as are the social networks to support individuals with rare diseases and their families.
Please follow the link to learn more about EURORDIS:
https://www.eurordis.org/
28th February 2020
Monitoring uptake of BRCA testing and equity - Published!
BRCA
testing received much publicity following Angelina Jolie’s editorial “My Medical Choice” in May 2013 and updated NICE clinical guidance (CG164) in June 2013.
We assessed the effect of these two concurrent events on BRCA
testing in one UK catchment area and relate this to socioeconomic deprivation. To examine the relative distribution of testing by deprivation, the deprivation status of patients who received testing was examined. Between April 2010 and March 2017, testing increased 11-fold and there was an 84% increase (P = 0.006) in BRCA
testing in the month following both publications. Further research is needed to examine differences in testing by the deprivation group which adjusts for confounders.
3rd May 2019
Time to review access to new cancer medicines in Europe?
The benefits of early access to medicines with high unmet need are recognised. In 2016 olaratumab received accelerated conditional approval from both the EMA and FDA for soft-tissue sarcoma, based on reduction in risk of death with an 11.8-month improvement in median overall survival in a phase II trial. However, failure to confirm these benefits in the post-authorisation pivotal trial has highlighted concerns regarding early access and conditional approvals.
Closer collaboration between multiple-stakeholders is needed to enhance the generation of rapid and comparative confirmatory trials are required. Early access systems may be improved by greater co-operation between countries regarding the collection of data in routine clinical care, and further research on post-marketing data analysis and interpretation, may also contribute to improved appraisal and continued access to new medicines.
7th November 2019
Monitoring uptake of BRCA testing and equity - Published!
BRCA
testing received much publicity following Angelina Jolie’s editorial “My Medical Choice” in May 2013 and updated NICE clinical guidance (CG164) in June 2013.
We assessed the effect of these two concurrent events on BRCA
testing in one UK catchment area and relate this to socioeconomic deprivation. To examine the relative distribution of testing by deprivation, the deprivation status of patients who received testing was examined. Between April 2010 and March 2017, testing increased 11-fold and there was an 84% increase (P = 0.006) in BRCA
testing in the month following both publications. Further research is needed to examine differences in testing by the deprivation group which adjusts for confounders.
3rd May 2019
Time to review access to new cancer medicines in Europe?
The benefits of early access to medicines with high unmet need are recognised. In 2016 olaratumab received accelerated conditional approval from both the EMA and FDA for soft-tissue sarcoma, based on reduction in risk of death with an 11.8-month improvement in median overall survival in a phase II trial. However, failure to confirm these benefits in the post-authorisation pivotal trial has highlighted concerns regarding early access and conditional approvals.
Closer collaboration between multiple-stakeholders is needed to enhance the generation of rapid and comparative confirmatory trials are required. Early access systems may be improved by greater co-operation between countries regarding the collection of data in routine clinical care, and further research on post-marketing data analysis and interpretation, may also contribute to improved appraisal and continued access to new medicines.
7th November 2019
Published opinion: Joint regulation for HTA in Europe?
The European Commission published a Proposal for a Regulation on Health Technology Assessment (HTA): ‘Proposal for a Regulation on health technology assessment and amending Directive 2011/24/EU’. A number of stakeholders, including some Member States, welcomed this initiative as it was considered to improve collaboration, reduce duplication and improve efficiency. There were however a number of concerns including its legal basis, the establishment of a single managing authority, the preservation of national jurisdiction over HTA decision-making and the voluntary/mandatory uptake of joint assessments by Member States.
Consolidated views on the Proposal are described by policy makers, payers, pricing and reimbursement authorities and academics. Expert commentary: The Proposal has since been extensively discussed at Council and while good progress has been achieved, there are still divergent positions. The European Parliament gave a number of recommendations for amendments. If the Proposal is approved, it is important that a balanced, improved outcome is achieved for all stakeholders. If not approved, the extensive contribution and progress attained should be sustained and preserved, and the best alternative solutions found.
15th February 2019
Review published: economic evaluations of targeted physical activity and sedentary behaviour interventions
The burden of noncommunicable diseases (NCD) on health systems worldwide is substantial. Physical inactivity and sedentary behaviour are major risk factors for NCD. A systematic review was carried out to examine how economic evaluations (EE) have addressed methodological challenges.
Fifteen EEs from four high-income countries were retrieved; there is a dearth of studies targeting sedentary behaviour as an independent risk factor from physical activity. Comparability of studies from the healthcare and societal perspectives were limited due to analysts’ choice in cost categories, valuation technique and time horizon differing substantially. The scarcity of and inconsistencies across EEs for these two behaviours have exposed a mismatch between calls for more preventative action to tackle NCD and the lack of information available on how resources may be optimally allocated in practice. Consequently, this paper offers a table of recommendations on how future studies can be improved.
10th July 2019
Published opinion: Joint regulation for HTA in Europe?
The European Commission published a Proposal for a Regulation on Health Technology Assessment (HTA): ‘Proposal for a Regulation on health technology assessment and amending Directive 2011/24/EU’. A number of stakeholders, including some Member States, welcomed this initiative as it was considered to improve collaboration, reduce duplication and improve efficiency. There were however a number of concerns including its legal basis, the establishment of a single managing authority, the preservation of national jurisdiction over HTA decision-making and the voluntary/mandatory uptake of joint assessments by Member States.
Consolidated views on the Proposal are described by policy makers, payers, pricing and reimbursement authorities and academics. Expert commentary: The Proposal has since been extensively discussed at Council and while good progress has been achieved, there are still divergent positions. The European Parliament gave a number of recommendations for amendments. If the Proposal is approved, it is important that a balanced, improved outcome is achieved for all stakeholders. If not approved, the extensive contribution and progress attained should be sustained and preserved, and the best alternative solutions found.
15th February 2019
Review published: economic evaluations of targeted physical activity and sedentary behaviour interventions
The burden of noncommunicable diseases (NCD) on health systems worldwide is substantial. Physical inactivity and sedentary behaviour are major risk factors for NCD. A systematic review was carried out to examine how economic evaluations (EE) have addressed methodological challenges.
Fifteen EEs from four high-income countries were retrieved; there is a dearth of studies targeting sedentary behaviour as an independent risk factor from physical activity. Comparability of studies from the healthcare and societal perspectives were limited due to analysts’ choice in cost categories, valuation technique and time horizon differing substantially. The scarcity of and inconsistencies across EEs for these two behaviours have exposed a mismatch between calls for more preventative action to tackle NCD and the lack of information available on how resources may be optimally allocated in practice. Consequently, this paper offers a table of recommendations on how future studies can be improved.
10th July 2019
Contact us today to learn more about how we can help you
Navigation
Services
Legal
© 2024
QC Medica LLP
Contact
QC Medica
Avenue HQ, 17 Mann Island
Liverpool, L3 1BP
Tel: +44 (0)151 440 2647
Email: info@qcmedica.com